Edmund R.S. KUNJI, PhD and Sotiria (Roula) TAVOULARI, PhD
MRC Mitochondrial Biology Unit, University of Cambridge, United Kingdom
Bioenergetic, metabolic and morphological consequences of citrin pathogenic variants on liver cells and tissue
Citrin deficiency is caused by the absence or malfunction of the transport protein citrin. Citrin resides in the membrane of mitochondria, special compartments of the human cells, where transport and breakdown of substances is used for the production of energy and other cellular functions. Citrin is specifically used for the import of the amino acid glutamate into the mitochondrion and the export of the amino acid aspartate, an important process in human metabolism. In citrin deficiency, citrin is absent or impaired through specific mutations leading to a defective metabolism, affecting primarily the liver. We study how these mutations affect the function of the protein and how this in turn impacts metabolic processes, energy production and the overall function of mitochondria and human cells. Our aim is to understand the basic mechanisms resulting from the dysfunction of the citrin protein in order to explore new therapeutic avenues.
Scientific summary
Citrin deficiency is an autosomal recessive disease caused by mutations in the SLC25A13 gene, which encodes for the mitochondrial aspartate/glutamate carrier isoform 2 (AGC2). A great variety of pathogenic variants have been reported in citrin deficiency, including missense and nonsense mutations, deletions and insertions as well as splice site variants. Our studies aim to unravel the molecular and cellular mechanisms of citrin dysfunction caused by these mutations in order to explain the diversity of symptoms and disease onset observed between citrin deficiency patients and to facilitate the development of new therapeutic strategies. We are following a multidisciplinary approach utilising a variety of biochemical, biophysical and cell biology techniques, including gene editing, advanced microscopy, metabolomic and bioenergetic measurements. We are developing novel cellular models of citrin deficiency to fully characterise the impact of pathogenic variants. Specifically, we are assessing the citrin variants for their functional, bioenergetic, metabolic and morphological consequences on the mitochondrion and their overall impact on cellular homeostasis. We are also investigating how nutritional supplements and novel therapeutic strategies could ameliorate the effects of citrin deficiency.
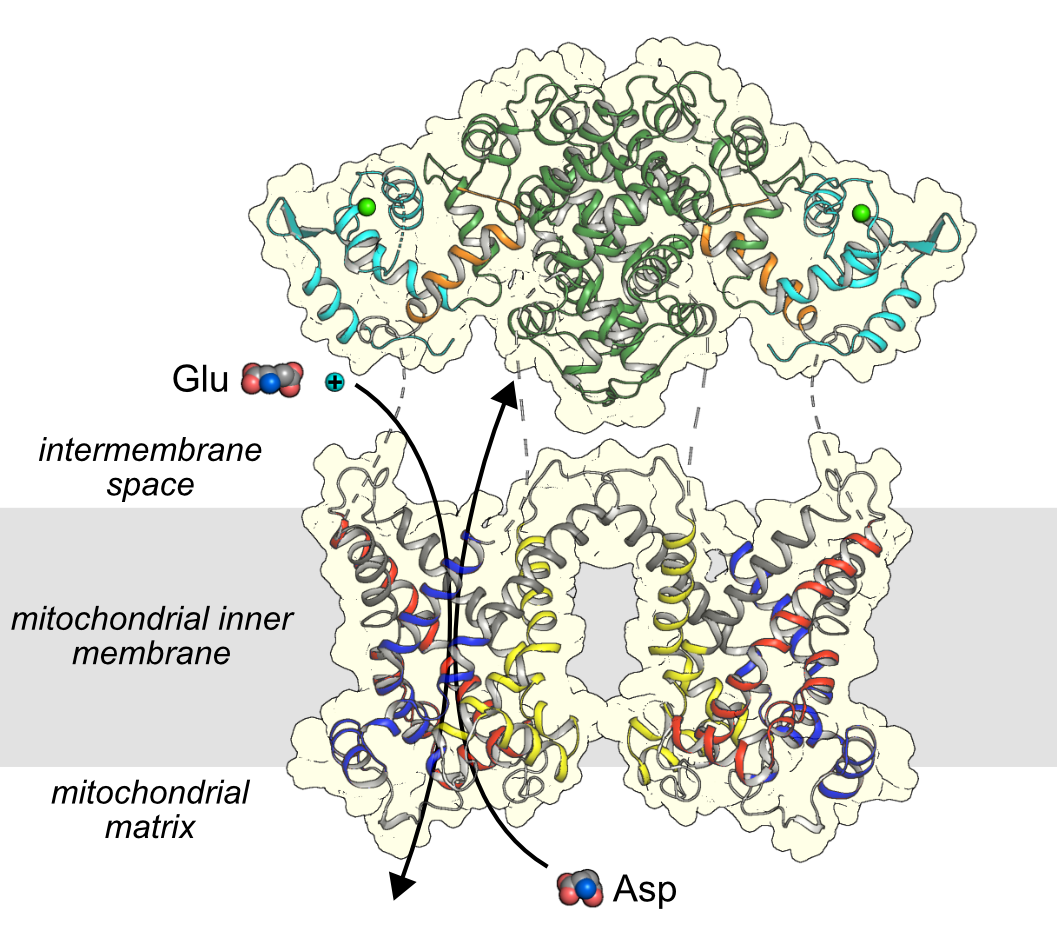
Citrin transporting aspartate and glutamate across the mitochondrial inner membrane
The N-terminal calcium regulatory domain of citrin has eight EF-hands with the calcium responsive domain colored in cyan and the static domain, which is involved in dimerization, in green. The carrier domain consists of three core elements shown in primary colors and three gate elements shown in grey. The C-terminal domain with an amphipathic helix is colored orange.
(Updated March 2022)


 Professor Edmund Kunji holds a PhD in Mathematics and Natural Sciences from the University of Groningen, The Netherlands. He carried out his post-doctoral studies in the group of Dr Richard Henderson at the MRC Laboratory of Molecular Biology, Cambridge. Since 2000, he is a programme leader at the MRC Mitochondrial Biology Unit, University of Cambridge, where he works with his team on the role of mitochondrial transport proteins in the translocation of metabolites, cofactors and inorganic ions across the mitochondrial inner membrane. Since 2019, he is Professor of Biophysics in Clinical Neurosciences of the University of Cambridge.
Professor Edmund Kunji holds a PhD in Mathematics and Natural Sciences from the University of Groningen, The Netherlands. He carried out his post-doctoral studies in the group of Dr Richard Henderson at the MRC Laboratory of Molecular Biology, Cambridge. Since 2000, he is a programme leader at the MRC Mitochondrial Biology Unit, University of Cambridge, where he works with his team on the role of mitochondrial transport proteins in the translocation of metabolites, cofactors and inorganic ions across the mitochondrial inner membrane. Since 2019, he is Professor of Biophysics in Clinical Neurosciences of the University of Cambridge.