Edmund R.S. KUNJI, PhD and Sotiria (Roula) TAVOULARI, PhD
MRC Mitochondrial Biology Unit, University of Cambridge, United Kingdom
Characterising the Role of the Human Aspartate/Glutamate Carrier in Citrin Deficiency
Citrin deficiency is caused by the malfunction of the transport protein citrin. Citrin resides in a special compartment of the human cell, called the mitochondrion. Normally, it is involved in the import of the amino acid glutamate into the mitochondrion and in the export of the amino acid aspartate, which is important for many cellular functions and for the function of the human body. However, through specific mutations the function of citrin can be impaired, which leads to the disease. We study how these mutations affect the function of the protein and how this in turn impacts the function of the mitochondrion and the human cell, all with the aim to explore new approaches to a cure.
Scientific summary
Citrin is one of the most complex of all transport proteins, as it consists of three separate domains. The first domain is the regulatory domain, which changes conformation upon binding or release of calcium. The second domain is a transporter domain, which transports the amino acid glutamate into the mitochondrion and aspartate out of the mitochondrion and the third domain binds to the regulatory domain in the calcium-bound state and is released in the calcium-free state as part of the regulatory process. Citrin also forms a dimer via the regulatory domain, and thus is composed in total of six dynamic domains. Our contribution to the project is the molecular characterisation of disease variants of citrin that are associated with citrin deficiency. We will also study the impact of citrin disease variants on cellular and mitochondrial function. Finally, we will analyse the metabolic effect of citrin deficiency and factors that may positively influence it in order to find a cure.
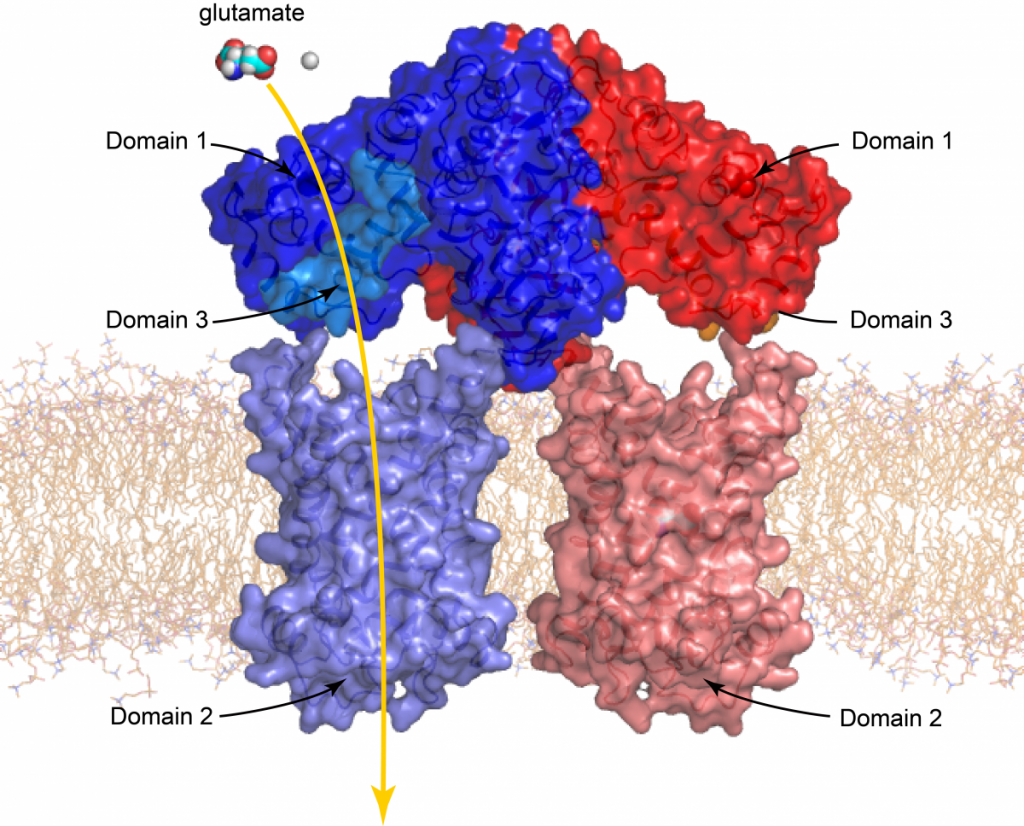
Basic structure of the mitochondrial aspartate/glutamate carrier (citrin)
Citrin consists of three domains. Domain 1 is the calcium regulatory domain, domain 2 the carrier domain and domain 3, an amphipathic helix, which is bound to the regulatory domain in the calcium-bound state. Two citrin proteins, one coloured blue and the other red, form together a dimer. Also shown is a glutamate molecule, which is transported by the carrier domain through the inner membrane together with a proton.

 Professor Edmund Kunji holds a PhD in Mathematics and Natural Sciences from the University of Groningen, The Netherlands. He carried out his post-doctoral studies in the group of Dr Richard Henderson at the MRC Laboratory of Molecular Biology, Cambridge. Since 2000, he is a programme leader at the MRC Mitochondrial Biology Unit, University of Cambridge, where he works with his team on the role of mitochondrial transport proteins in the translocation of metabolites, cofactors and inorganic ions across the mitochondrial inner membrane. Since 2019, he is Professor of Biophysics in Clinical Neurosciences of the University of Cambridge.
Professor Edmund Kunji holds a PhD in Mathematics and Natural Sciences from the University of Groningen, The Netherlands. He carried out his post-doctoral studies in the group of Dr Richard Henderson at the MRC Laboratory of Molecular Biology, Cambridge. Since 2000, he is a programme leader at the MRC Mitochondrial Biology Unit, University of Cambridge, where he works with his team on the role of mitochondrial transport proteins in the translocation of metabolites, cofactors and inorganic ions across the mitochondrial inner membrane. Since 2019, he is Professor of Biophysics in Clinical Neurosciences of the University of Cambridge.